Together with the CE Marking it is fundamental obligation of the manufacturer to furnish information for every device that is set on the market. The necessary information to identify the product, to guarantee a safe use of it, to allow the identification of the manufacturer, to track the product down, is brought on the label and when necessary, in the instructions for use. Label and instructions for use are furnished by Leone in several languages.
It is of fundamental importance that all the information that the manufacturer has meant for the product, that is shown up on the device itself and/or on the single packing or, eventually, on the commercial packing, is maintained during all the marketing steps and reaches integral the end users.
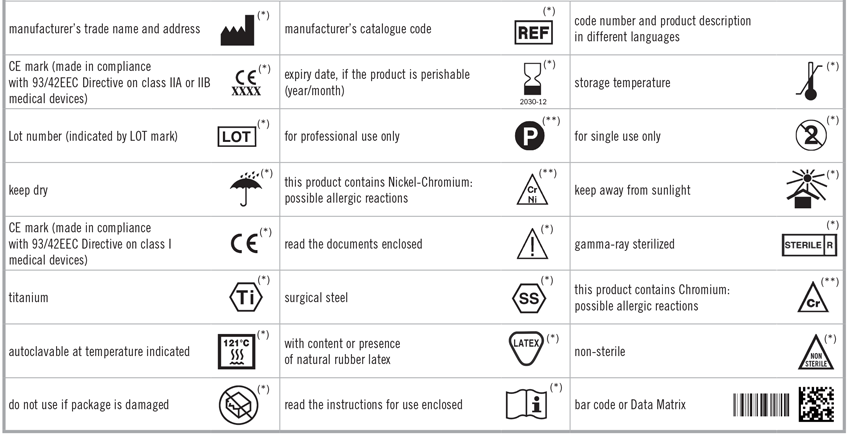
The label on the package of any medical device set on the market will show the symbols in compilance with the harmonized standards.
The symbols marked with a single (*) are based on the ISO 21531, ISO 15223-1, EN 980 European Standard and on the 93/42EEC Directive.
The symbols marked with double (**) have instead been performed by us.
Leone products are intended exclusively for orthodontic, implantology and dental use and should only be used by skilled and licensed professionals, who will be held the sole responsible for the construction and the application of prostheses, partially of fully manufactured with the above mentioned products. For a proper usage of the devices, it is recommended to use them in conjunction with other original Leone products, accessories and instruments. With the exception for the instruments, all products are designed and manufactured for single use only. After removal from the patient's mouth, they must be disposed of properly and according to the law in force. We accept no responsibility for any damage, injury or other damage caused by reuse of declared single-use products. Decontaminate and sterilize the products that have come into contact with a different patient. The effects of the metallic orthodontic and implantology devices should be carefully monitored during MRI (Magnetic Resonance Imaging). It is recommended to instruct the patient to report in advance the presence of any type of device and dental materials to the radiologist staff in charge of the MRI. Ceramic and polymeric products (with the exception for certain articles containing medical grade Barium) are not radiopaque.